The research in the Werz lab is focused on synthetic organic chemistry in broad terms. We are interested in the development and application of elegant and efficient synthetic and catalytic methods, and on the total syntheses of larger natural and non-natural products (e.g. in the field of carbohydrates, glycolipids and fluorescent dyes).
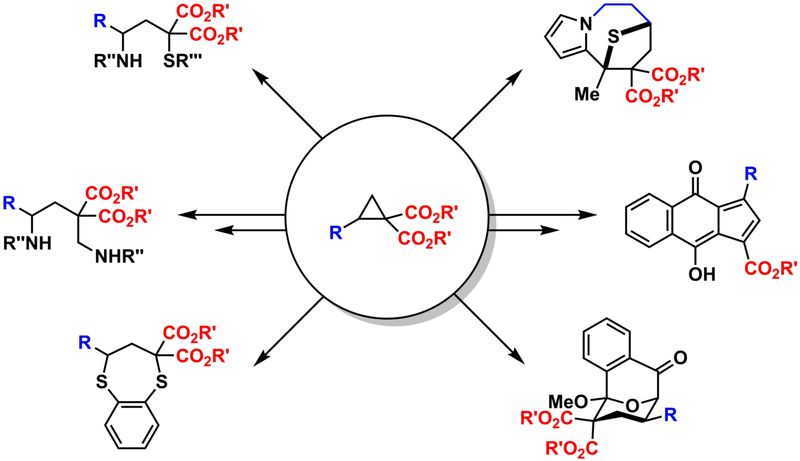
Donor-Acceptor-Cyclopropanes
The weak bond between vicinal donors and acceptors in a cyclopropane paves the way for a plethora of unusual reactions. Guided by quantum chemical studies1 we designed methods to obtain oligoacetals2,3 and spiroketals4 starting from respective cyclopropanes. These results were the basis to develop also routes from furan to 3,3‘-linked bispyrrols and larger oligomers.5 Similar transformations employing chalcogen-transferring agents yielded 3,3‘-linked bisthiophenes or corresponding cage-like compounds.6
 Cyclopropanes with geminal diester units have been used in a variety of (3+n)-cycloaddition and ring-opening reactions. For the latter special emphasis is put to the attachment of two different residues to 1- and 3-position (and none of them must be H).7,8 In the field of cycloaddition reactions four-, five, six- and seven-membered ring systems have been generated.9,10 Special attention is given to synergistic catalytic approaches where a Lewis acid activates the cyclopropane and another type of catalyst generates a fleeting intermediate as reaction partner.11,12 Kinetic studies have provided a deeper understanding which structural units are important for the reactivity of these types of cyclopropanes.13
Cyclopropanes with geminal diester units have been used in a variety of (3+n)-cycloaddition and ring-opening reactions. For the latter special emphasis is put to the attachment of two different residues to 1- and 3-position (and none of them must be H).7,8 In the field of cycloaddition reactions four-, five, six- and seven-membered ring systems have been generated.9,10 Special attention is given to synergistic catalytic approaches where a Lewis acid activates the cyclopropane and another type of catalyst generates a fleeting intermediate as reaction partner.11,12 Kinetic studies have provided a deeper understanding which structural units are important for the reactivity of these types of cyclopropanes.13
[1] T. F. Schneider, D. B. Werz, Org. Lett. 2011, 13, 1848-1851.
[2] T. F. Schneider, J. Kaschel, B. Dittrich, D. B. Werz, Org. Lett. 2009, 11, 2317-2320.
[3] T. F. Schneider, J. Kaschel, S. I. Awan, B. Dittrich, D. B. Werz, Chem. Eur. J. 2010, 16, 11276-11288.
[4] C. Brand, G. Rauch, M. Zanoni, B. Dittrich, D. B. Werz, J. Org. Chem. 2009, 74, 8779-8786.
[5] J. Kaschel, T. F. Schneider, D. Kratzert, D. Stalke, D. B. Werz, Angew. Chem. Int. Ed. 2012, 51, 11153-11156.
[6] J. Kaschel, C. D. Schmidt, M. Mumby, D. Kratzert, D. Stalke, D. B. Werz, Chem. Commun. 2013, 49, 4403-4405.
[7] L. K. B. Garve, P. G. Jones, D. B. Werz, Angew. Chem. Int. Ed. 2017, 56, 9226-9230.
[8] A. U. Augustin, P. G. Jones, D. B. Werz, Chem. Eur. J. 2019, 25, ASAP.
[9] A. U. Augustin, M. Sensse, P. G. Jones, D. B. Werz, Angew. Chem. Int. Ed. 2017, 56, 14293-14296.
[10] L. K. B. Garve, M. Pawliczek, J. Wallbaum, P. G. Jones, D. B. Werz, Chem. Eur. J. 2016, 22, 521-525.
[11] A. Lücht, L. J. Patalag, A. U. Augustin, P. G. Jones, D. B. Werz, Angew. Chem. Int. Ed. 2017, 56, 10587-10591.
[12] M. Petzold, P. G. Jones, D. B. Werz, Angew. Chem. Int. Ed. 2019, 58, 6225-6229.
[13] A. Kreft, Alexander Lücht, J. Grunenberg, P. G. Jones, D. B. Werz, Angew. Chem. Int. Ed. 2019, 58, 1955-1959.
Carbopalladations Cascades
Highly efficient Pd-catalyzed domino sequences are applied for the synthesis of chromans and isochromans starting from carbohydrates.1,2 In such a way, stereochemical information of the carbohydrate is transferred to the heterocyclic system. Similarly, persubstituted biphenyls are generated in one step.3 Related sequences starting with appropriately substituted arenes furnish interesting π systems in a short and efficient manner,4 e.g. dibenzopentafulvalenes were accessed by a quadruple domino carbopalladation sequence.5
Besides common syn-carbopalladations we designed an anti-carbopalladation.6,7 There, the two residues are attached at opposite sides of the emerging double bond. Different terminating processes are feasible such as Heck reactions, Stille couplings or C-H activations.8 In addition, also terminations with heteratoms have been successfully carried out, e.g. affording tetrasubstituted enol ethers.9 The synthetic power of such a anti-carbopalladation cascade has shown, e.g. by a total synthesis of lysergic acid.10

[1] M. Leibeling, D. C. Koester, M. Pawliczek, S. C. Schild, D. B. Werz, Nature Chem. Biol. 2010, 6, 199-201.
[2] M. Leibeling, B. Milde, D. Kratzert, D. Stalke, D. B. Werz, Chem. Eur. J. 2011, 17, 9888-9892.
[3] M. Leibeling, D. B. Werz, Chem. Eur. J. 2012, 18, 6138-6141.
[4] M. Leibeling, M. Pawliczek, D. Kratzert, D. Stalke, D. B. Werz, Org. Lett. 2012, 14, 346-349.
[5] J. Wallbaum, R. Neufeld, D. Stalke, D. B. Werz, Angew. Chem. Int. Ed. 2013, 52, 13243-13246.
[6] M. Pawliczek, T. F. Schneider, C. Maaß, D. Stalke, D. B. Werz, Angew. Chem. Int. Ed. 2015, 54, 4119-4123.
[7] T. Schitter, A. Reding, D. B. Werz, Synlett 2019, 30, 1275-1288.
[8] A. Reding, P. G. Jones, D. B. Werz, Angew. Chem. Int. Ed. 2018, 57, 10610-10614.
[9] T. Schitter, P. G. Jones, D. B. Werz, Chem. Eur. J. 2018, 24, 13446-13449.
[10] B. Milde, M. Pawliczek, P. G. Jones, D. B. Werz, Org. Lett. 2017, 19, 1914-1917.
Carbohydrate and Glycolipid Chemistry
In the field of carbohydrate chemistry the WERZ research group is active in the preparation and biological evaluation of bacterial carbohydrates as well as mammalian glycolipids. Different questions (sometimes studied in collaboration) are in the focus.
As part of the Collaborative Research Center on membranes we are interested in the tailor-made assembly of glycosphingolipids with variable hydrocarbon chains by using a modular chemical approach. Next to their biological relevance a major aim of this project is to get insights into the fundamental processes of membrane domain formation.1 The use of a new class of fluorescent dyes2 with minimal impact on the structural integrity of membrane domains allows the visualization of lateral organization and fate of membrane structures in transport processes and fusion assays of glycolipid-rich membranes. Another approach use the attachment of fluorescent dyes to the carbohydrate head group. A highly modular synthesis was designed that allows the assembly of several structurally related glycolipids which differ in their dyes, in the linker length and in the type of fatty acid.3 In collaboration in-depth biophysical studies related to the membrane organization are performed.4
In collaboration with biophysicists force constants between single oligosaccharides derived from the marine sponge Microciona prolifera were measured and served as model systems for multivalent interactions in early evolutionary stages of life.5

[1] O. M. Schütte, A. Ries, A. Orth, L. J. Patalag, W. Römer, C. Steinem, D. B. Werz, Chem. Sci. 2014, 5, 3104-3114.
[2] L. J. Patalag, D. B. Werz, J. Org. Chem. 2012, 77, 5297-5304.
[3] J. Sibold, K. Kettelhoit, L. Vuong, F. Liu, D. B. Werz, C. Steinem, Angew. Chem. Int. Ed. 2019, 58, ASAP.
[4] M. Bosse, J. Sibold, H. A. Scheidt, L. J. Patalag, K. Kettelhoit, A. Ries, D. B. Werz, C. Steinem, D. Huster, Phys. Chem. Chem. Phys. 2019, 21, 15630-15638.
[5] A. de Cienfuegos, M. Oelkers, E. Kriemen, C. Brand, M. Stephan, E. Sunnick, D. Yüksel, V. Kalsani, K. Kumar, D. B. Werz, A. Janshoff, J. Am. Chem. Soc. 2012, 134, 3326-3329.
Carbohydrate Mimics
Next to O-glycoside chemistry we are also involved in the synthesis of C-glycosides using a highly modular approach.1 In contrast to O-glycosidic bonds C-glycosidic bonds are not cleaved by hydrolysis or enzymolysis. Key reactions we use in our strategy are Pd-catalyzed coupling reactions allowing the efficient preparation of C- glycosidic bonds between different monosaccharide building blocks. Especially Pd-catalyzed Sonogashira and Stille reactions of alkynes or olefins in combination with 1-functionalized glycals were utilized. In a further step the native hydroxyl pattern is regenerated.2
 Spiroannelated cyclopropanes are another possibility to influence conformations of saccharides. We developed synthetic routes to attach a spiroannelated three-membered ring at the C-5 position.3,4 On one hand side, the inversion of the pyran ring is hampered, on the other the 6-hydroxyl group is precisely located to either the left or the right side of the cyclopropane. The attachment of the cyclopropyl subunit is the smallest possible modification, the structural integrity of the pyran is almost maintained. This chemistry can be easily extended to respective nucleic acid chemistry.5 Chemical transformations of the three-membered ring allow the facile access to 5-alkylated pyranose and 4-alkylated nucleic acid building blocks.6
Spiroannelated cyclopropanes are another possibility to influence conformations of saccharides. We developed synthetic routes to attach a spiroannelated three-membered ring at the C-5 position.3,4 On one hand side, the inversion of the pyran ring is hampered, on the other the 6-hydroxyl group is precisely located to either the left or the right side of the cyclopropane. The attachment of the cyclopropyl subunit is the smallest possible modification, the structural integrity of the pyran is almost maintained. This chemistry can be easily extended to respective nucleic acid chemistry.5 Chemical transformations of the three-membered ring allow the facile access to 5-alkylated pyranose and 4-alkylated nucleic acid building blocks.6

[1] D. C. Koester, A. Holkenbrink, D. B. Werz, Synthesis 2010, 3217-3242.
[2] D. C. Koester, E. Kriemen, D. B. Werz, Angew. Chem. Int. Ed. 2013, 52, 3059-3063.
[3] C. Brand, M. Granitzka, D. Stalke, D. B. Werz, Chem. Commun. 2011, 47, 10782-10784.
[4] C. Brand, K. Kettelhoit, D. B. Werz, Org. Lett. 2012, 14, 5126-5129.
[5] C. Köllmann, S. M. Wiechert, P. G. Jones, T. Pietschmann, D. B. Werz, Org. Lett. 2019, 21, 6966-6971.
[6] C. Köllmann, P. G. Jones, D. B. Werz, Org. Lett. 2018, 20, 1220-1223.
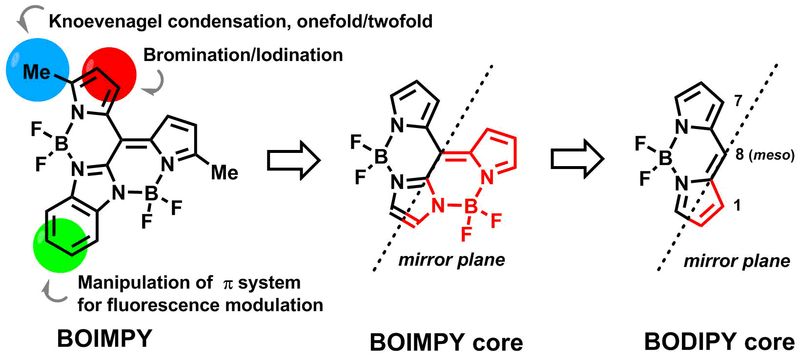
Novel Types of Fluorescent Dyes
Inspired by the well-known BODIPY motif a fast entry into a novel family of highly fluorescent fluorophores termed BOIMPYs was designed. Two BF2 units ensure an efficient exploitation of the meso position and trigger absorptions at ~ 600 nm. The structural relationship to well-studied BODIPYs enables common modes of post-functionalization to be easily transferable to the new motif.1-3
Furthermore, ethano-bridged BODIPY oligomers were prepared by a simple oxidative coupling reaction of the respective carbanions egnerated in situ. Dimers, trimers, tetramers, hexamers and octamers were obtained. Depending on the substitution pattern J-aggregate formation was observed affording strongly red-shifted superfluorophors with quantum yields up to unity.4
[1] L. J. Patalag, P. G. Jones, D. B. Werz, Angew. Chem. Int. Ed. 2016, 55, 13340-13344.
[2] L. J. Patalag, P. G. Jones, D. B. Werz, Chem. Eur. J. 2017, 23, 15903-15907.
[3] L. J. Patalag, M. Loch, P. G. Jones, D. B. Werz, J. Org. Chem. 2019, 84, 7804-7814.
[4] L. J. Patalag, L. Phong Ho, P. G. Jones, D. B. Werz, J. Am. Chem. Soc. 2017, 139, 15104-15113.

